GMP compliance in pharmaceutical manufacturing requires that any process, person, environment or equipment with direct impact on the quality and safety of the product being produced must operate within specified limits. These specified limits should be under the direct control of the manufacturing team, with countermeasures available in the event of a problem occurring. In addition to this, any other part of the production or storage processes that have an indirect impact must also be assessed for possible risk impact.
Humidity may not seem like an obvious cause of problems, or something that could even result in production being non-compliant, but this can and does occur. Many phenomena are influenced by the relative humidity level, and they can cause production processes to be less efficient, less predictable and more prone to producing out of specification products.
Some common issues that can arise from poor humidity control are:
- Increased energy consumption
|
- Altered aesthetic presentation
|
|
|
|
- Poor test equipment accuracy
|
|
- Changes in electrical conductivity
|
|
- Degradation of buildings and products
|
|
|
|
|
- Reduced refrigeration plant efficiency
|
|
|
|
|
The figure below shows the influence on storage of a variety of materials as relative humidity changes. Bear in mind that storage in this sense is not restricted to just warehousing but also applies to any material, object or equipment that is stationary in a fixed location for extended periods. So think of this in the context of equipment and fittings within a pharmaceutical manufacturing plant.
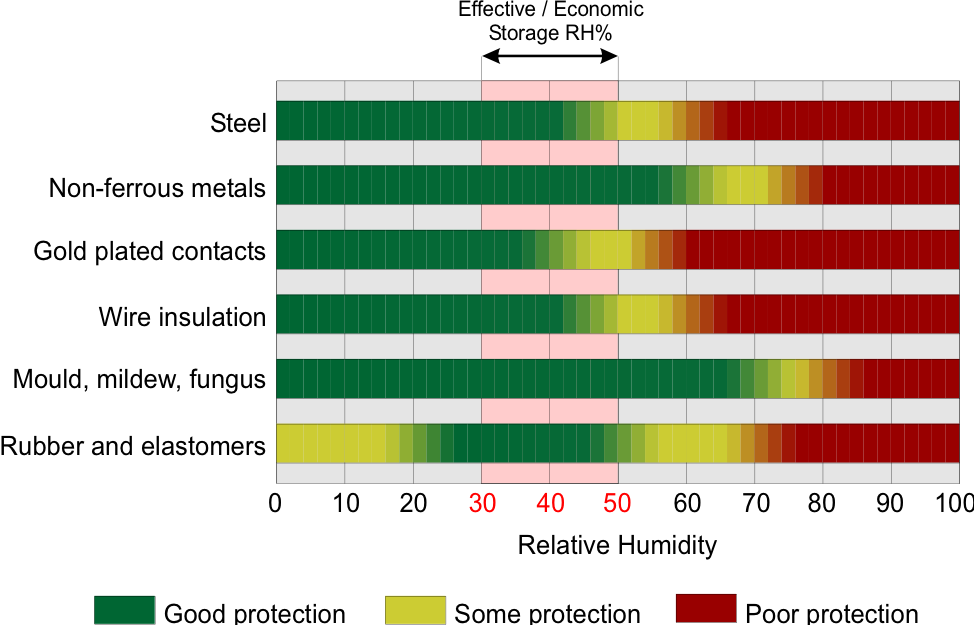
Figure 1. Effect of RH% on materials in static locations.
It is important to note the relationship between relative humidity (RH%) and air temperature, variations in temperature will also affect the RH%. For example, a sufficiently large drop in air temperature can result in water vapour condensing out. Alternatively, cold surfaces will cause condensation to form if the surface temperature drops below the dewpoint of the air around it.
Humidity in manufacturing areas
Looking at this from another angle, consider the following typical conditions found in areas related to solid dosage production:
|
Production area
|
Temperature
|
Humidity
|
|
Weighing, Mixing
|
20 to 22°C°
|
35 to 40% RH
|
|
Compression
|
20°C
|
25 to 35% RH
|
|
Pan coating
|
12 to 95°C
|
10 to 70%
|
|
Filling and Packing
|
20°C
|
10 to 35% RH
|
|
Storage
|
20 to 25°C
|
45% RH
|
From the table above, if the RH% levels therein are correctly maintained for the designated process and surrounding areas then no humidity related issues are likely to occur, either to the material being produced or the production equipment itself.
Of course, the actual required RH% values for any given process are dependent on many factors:
- The materials being used and their sorption isotherms, hygroscopicity and sensitivity to water.
- The manufacturing space itself: Volume, building insulation, airflow control, number of openings, geographic location, climate, etc.
- SOPs and operator behaviour: Cleaning cycles, procedures for material transfers, etc.
- GMP specified values which may be influenced by other factors.
Therefore, in all areas of production, humidity levels should always be carefully considered, not just to suit the material being produced but to also take into account any other effects arising from unexpected RH% levels that could occur and potentially disrupt production.
So, the questions that pharmaceutical manufacturers have to ask themselves are:
- Do the relative humidity levels within my facility exceed GMP specified values at any time?
- If they do, for how long? What is the recovery time to return to the specified value?
- What is the impact of this excursion from the specified value and do I need to react to this?
- Is recovery time fixed or variable? For example, with effective humidity control the recovery will be quicker in dry winter months or longer during wet, humid periods.
Bearing all of the preceding points in mind, take a moment to ask yourself if you are experiencing moisture related production or quality problems in your production areas:
- Maybe the flow of API and excipients is not as expected, it could be either too fast or too slow depending on how moisture affects the materials.
- The Angle of Repose in powder or granulate samples is not as expected, if a small amount of available water is able to bridge the gaps between particles, electrostatic attraction of the water to surfaces will alter the Angle of Repose, another sign that flow characteristics may have changed.
- Do you experience any variations when weighing of batches of raw materials or finished product that could be caused by fluctuations in water content? This can be determined from referring to the sorption isotherms of the materials being weighed.
- Are there signs of clogging, caking or other obstructions in silos, bins, pipework and process equipment? Does pneumatic transport take longer than expected? Is there additional noise and vibration that might be attributed to blockages. It’s also important to remember that the increased air pressure of pneumatic transport systems will affect the dewpoint of the air within the system, and condensation could occur at higher than expected temperatures.
- When using moisture-permeable containers for packaging (plastic bags and semi-rigid, low-density polyethylene (LDPE) pouches for large volume parenterals (LVPs), and LDPE ampoules, bottles, and vials), due consideration should be given to the stability of the contents under high humidity conditions.
- Moisture may have an undesirable effect on chemical stability (e.g. some antibiotics may undergo hydrolysis) and physical stability (e.g. dissolution rate may change). Drugs with functional groups such as esters, amides, lactones or lactams may be susceptible to hydrolytic degradation which is a commonly encountered mode of drug degradation. Many polymers that are susceptible to hydrolysis, for example, the polyesters PLA and PLG, degrade by random hydrolysis that takes place homogeneously throughout the bulk of the polymer device.
- Consider the impact of moisture in bulk materials and finished product.
Even when all of these factors are taken into account, more radical action might need to be considered. Perhaps you need to go right back to the start and ask, “How was the RH% level for production and storage processes determined in the R&D phase?” Because it can sometimes be that the environmental conditions for producing a new product are adopted from a similar, existing product already in production based on the assumptions that:
- These conditions work and are proven, and there does not appear to be any significant dissimilarities between the two products.
- Particularly in cases where an existing manufacturing area is being repurposed for a new product, the available HVAC system can produce those conditions.
Storage and Warehousing
Deviations from the desired temperature and humidity conditions must be minimised, controlled and documented. Unfortunately, temperature and humidity excursions are almost inevitable, but automated control of HVAC and humidity control systems will improve response and recovery, and can also provide historical and trend data to track these excursions.
Although minor deviations probably have no significant impact, however the effects temperature or humidity deviations on every item held in storage need to be considered. This can be a daunting task, as it is not unusual for warehouses to contain hundreds or maybe even thousands of different inventory items. It is also worth considering that new items will be added in future, which will require some form of assessment. The impact assessment therefore has the potential to become an enormous task.
It is easier to ensure temperature and humidity are controlled within defined limits (e.g. 22°C @ 50%R.H.) supported by automatically generated logs..
Psychrometrics and humidity control
There are different ways to control humidity, or more correctly, to attempt to control humidity.
Using outdoor air for ventilation - Ventilation air must have a lower moisture content than the air within the building to be effective and is therefore at the mercy of changing weather and seasonal conditions.
For the most part, we should ignore using untreated outdoor air because of its variability. So let us look at other ways to treat the air either entering or already within the building.
Heating
This will lower the relative humidity but absolute humidity remains unchanged, there is still the same mass of water vapour and the dewpoint is unchanged. This might be a reasonable humidity control strategy if you need to heat the area for comfort, but in energy terms it can be relatively expensive.
Cooling
Using cooling coils to reduce the air temperature below its dewpoint will lower the RH% after cold air is re-heated, and it will also reduce absolute humidity. However, efficiency falls significantly at air temperatures below 10°C. In addition to this, the inevitable condensation that forms on the cooling coils can become a maintenance issue if they are prone to corrosion. Finally, the wet conditions are a good breeding ground for bacteria and mould, which are definitely not wanted anywhere near pharmaceutical production.
Desiccant dehumidification
This reduces both relative and absolute humidity, and also reduces dew-point while not being temperature sensitive (operating range is between +40°C to -40°C). Lower airflows can be used when compared to other methods of air treatment, resulting in energy savings. This form of humidity control is also very flexible when considering using multiple energy sources e.g. gas, steam, LPHW, etc. so available utilities and waste heat can be used. The system runs dry which reduces the possibility of microbial growth and maintenance arising from wet conditions, which can also translate to longer equipment life. Furthermore, this form of humidity control can dry down to a -70°C dewpoint which may be required for sensitive APIs.
In closing
With over 60 years of experience in pharmaceutical and other areas of manufacturing, Munters can provide desiccant dehumidification solutions as well as expertise and support to ensure your production remains GMP compliant. We are able to provide scalable systems that can be deployed in R&D, pilot plant and full scale manufacturing areas, ensuring that your investments of time and effort can be leveraged as you move from limited production for testing and trials through to full production.


