エラーが発生しました。後でもう一度試して下さい。
商業分野
サインイン
マーケット
ソリューション
エラーが発生しました。後でもう一度試して下さい。
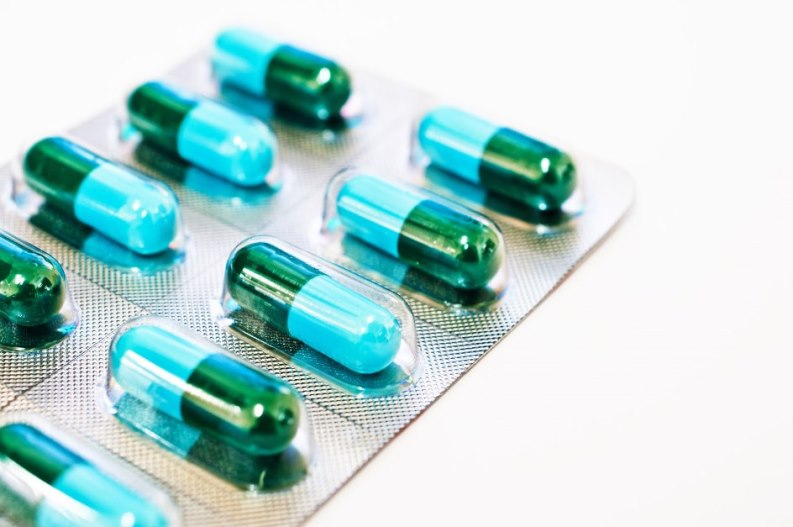
The February 2020 issue of Processing magazine features Martin Ginty’s white paper, “The Perfect Climate for Capsule Storage.”
Ginty, Global Pharmaceutical Industry Manager for Munters, discusses the significant role humidity control plays in achieving quality targets during pharmaceutical processing. Ginty writes, "One thing that has already changed and will also continue to develop, is the need to produce capsules with consistent quality characteristics, particularly when producing GMP-compliant products."
If the air is too dry, gelatine capsules become brittle. In addition, the potential for gelatine to undergo cross-linking and hydrothermal contraction increases leading to product quality issues. However, if relative humidity levels are too low, capsules may stick to each other and be difficult to package properly. Yet, with accurate control of relative humidity levels, these problems can be avoided and product quality will benefit as a result.
Read the article.
ユーザーIDが正しくありません。
エラーが発生しました。後ほどもう一度お試しください。
パスワードをお忘れですか?
ユーザーIDが正しくありません。
エラーが発生しました。後ほどもう一度お試しください。
Check your email within a couple of minutes to reset your password, if you can’t see any incoming messages try to check into your spam folder!