Humidity could be a significant risk to your manufacturing process
Pharmaceutical ingredients and products are often extremely hygroscopic and are easily damaged by excess moisture. Uncontrolled humidity in production can also lead to dangerous mold and bacterial growth, contaminated or poor-quality products, and serious compliance issues.
The production of critical formulations in pharmaceutical and nutraceutical manufacturing requires precise temperature and humidity control. These environments are extremely sensitive to ambient moisture. Exposure to moist air can cause products to deteriorate, stick together, soften, break apart, or become susceptible to microbial growth. Machines and pipes in silos and conveying lines may become clogged, impeding production, transport, and storage, as well as resulting in increased maintenance and product waste. Desiccant dehumidification can help prevent costly, time-intensive problems.
With uncontrolled humidity you risk:
- Product quality and safety
- Maintaining GMP and regulatory compliance
- Unexpected downtime
- Compromised active pharmaceutical ingredients (API) efficacy

“Without dehumidification, even the slightest amount of excess moisture will cause ice to build on the specimens. This is a fundamental barrier to producing quality electron microscopic images. If the resolution is poor, we won’t get the image and won’t solve what we need to.”
Dr. Chris Russo, Laboratory of Molecular Biology
Medical Research Council


Optimal dehumidification is essential in pharmaceutical processing
Without creating the perfect climate, every step of the manufacturing process can be negatively affected.
In a humid environment, raw materials will clump and stick during storage and conveying, resulting in product waste and excessive cleaning. Dosing and granulation during weighing, mixing, or blending, can be compromised due to ambient moisture.
In cleanrooms, laminar flow cabinets, and R&D labs, uncontrolled humidity can affect test result reproducibility, and will negatively impact the environment for the people working on the premises. In an optimal and stable environment, product drying times in fluid beds and coaters will be reduced, while the production output will increase in spray drying.
During filling and packing, any type of moisture can harm product quality, reduce shelf life, and in the worst case cause production stoppages. Excessive moisture in the cold chain creates frost, ice, and dangerous conditions for the workers. Moisture is a hidden enemy in all parts of the process, risking production efficiency, worker safety, and product quality.
Munters has decades of experience in manufacturing desiccant dehumidifiers and climate control systems for the pharma industry. Our solutions are available for every area of production, from small R&D labs to large clean rooms and commercial processing lines.

The benefits of a desiccant dehumidifier
The most effective way to control moisture in your manufacturing facility, laboratory, or clean room, is with a reliable desiccant dehumidifier.
- Year-round, precise humidity and moisture control
- Maintain industry standards and regulatory compliance
- Maintain product efficacy and shelf life
- Reduce the risk of mold or bacteria growth
- Eliminate condensation
- Prevent ingredient or product clumping and sticking
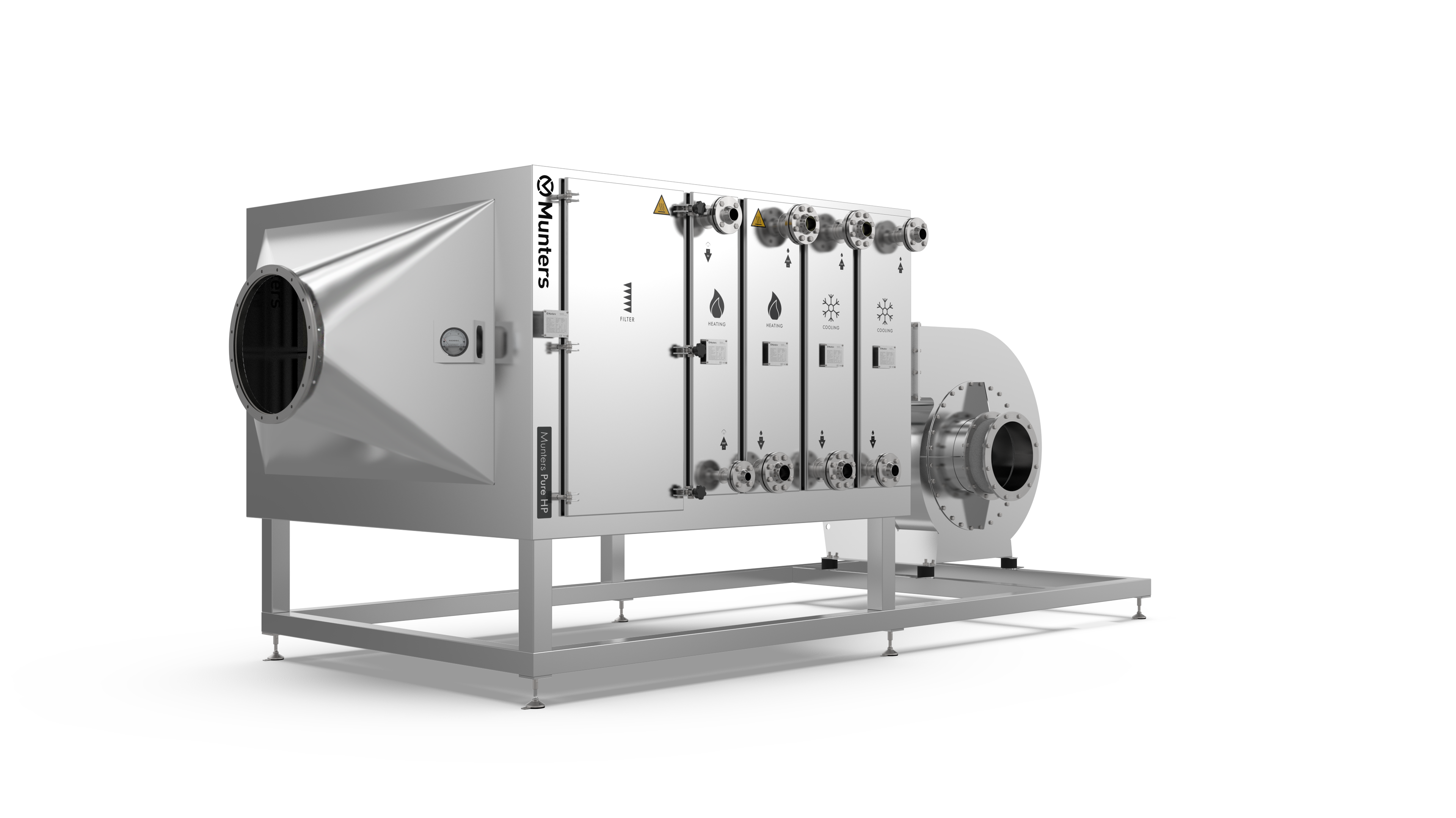
Product highlights
Within pharma processes, the requirements for humidity control are specific and it is critical to keep the moist at optimal levels all year round. Regardless of where in the process, product quality must be ensured and safety guaranteed. Munters ML dehumidifiers are highly flexible and suitable for wide range of pharma applications. These precise all-rounders for desiccant dehumidification are offering:
- Availability in smaller to larger size with possibility to add condensation as well as gas an steam generation units, depending on actual size
- Easy installation and maintenance thanks to optimized access panels
- Airflow in a range from 180 m3/h to 3,000 m3/h.
Comprehensive solutions for compliance and cleanrooms
More products for the pharmaceutical industry
Case studies
Learn more about humidity control in pharmaceutical applications
Service & maintenance for seamless operations
In pharmaceutical and nutraceutical production, as well as in laboratories, an unplanned production stop can cause severe damage to the product quality or test results, and be very costly.
Munters equipment is always backed by our world-leading service team. They are directly employed by Munters, with decades of experience in maintaining our climate control systems. With a Munters Service Agreement you can extend your dehumidification system's life, increase efficiency, minimize downtime, and ensure your production facilities will always stay regulatory compliant.